
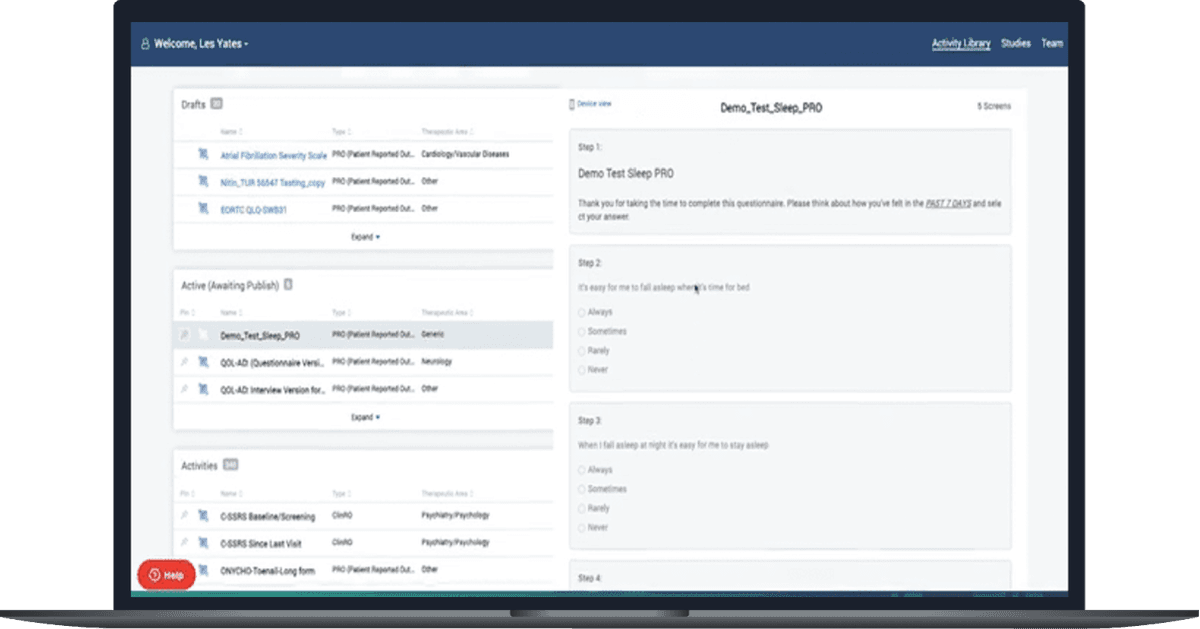
A Full Library of Assessments
Seamlessly Meet eCOA Regulation Compliance
Increase participant compliance and study data quality while meeting regulatory requirements worldwide.
Comprehensive eCOA Library
Our 545+ eCOAs within our library are pre-vetted, tested, and streamlined for licensing procurement, reducing the factors that lead to study startup delays and allowing for faster cycle times overall.
With THREAD, There Are No eCOA Compromises
One continuous flow of data from one unified platform. THREAD provides configurable data collection methods with configurable options including smart reminders, alert workflows, and instant data visualization.
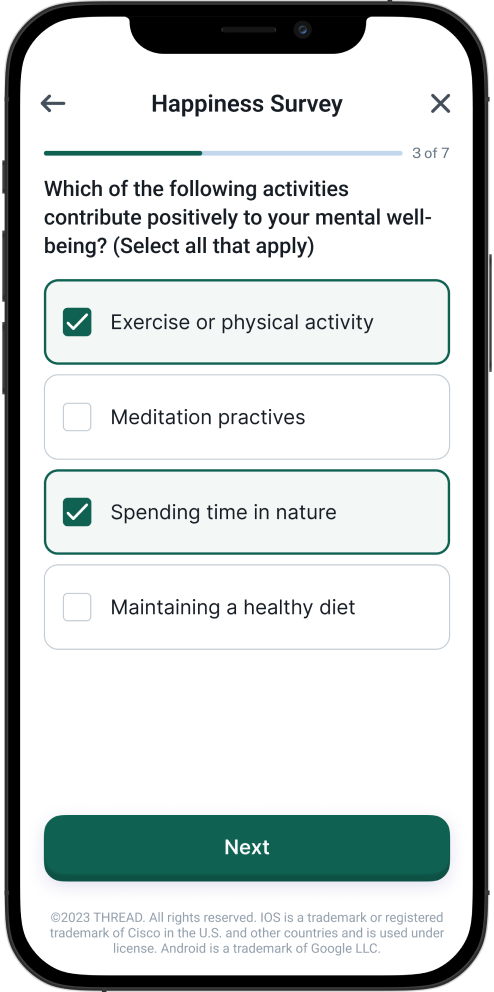
Electronic Patient Reported Outcomes
(ePROs) for validated assessments completed directly by the participant.
Clinician Reported Outcomes
(ClinRos) for validated assessments to be completed by the site staff and/or eCOA raters.
Sensors
For prebuilt integrations with hundreds of 510(k) cleared and CE Mark approved medical devices, wearables, and health apps.
Surveys
For non-validated surveys, diaries, brief activities, safety signaling, and medication confirmations.
Electronic Source Forms (eSource)
For document completion by site staff, home health organizations, and eCOA raters.
Electronic Case Report Forms (eCRFs)
For completion by site staff and other stakeholders during on-site and/or virtual visits.
Electronic Device Reported Outcomes (eDROs)
For device + activity measurements.
