
Patient and HCP Listening
Listen to Real Patient Voices

How it Works
Patient and HCP Listening takes a novel approach to collect real patient and caregiver voices rapidly to co-create and mitigate risks throughout the clinical study lifecycle.
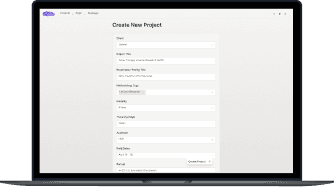
1
Configure a Mixed Methods Study

2
Access Real Patients
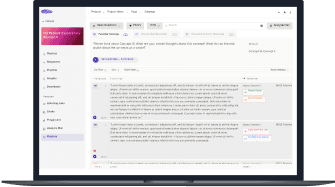
3
Analyze with Linguistic Experts & AI
Filter by
Not all-inclusive. inVibe is experienced in over 150 unique indications.
No patient voices found that match your filter criteria