
Protecting the Patient Journey
Optimize Study Performance by Defining the User Journey
THREAD has set a new industry standard for technology best-practices in clinical research we call “Define”. We start every study by defining the journey of platform users. This means mapping study requirements to the software and services that enable patient-centered and site-centered technology deployment.
Our approach includes a thorough consultative protocol analysis and generation of a detailed Journey Map. These assets enable Sponsors and CRO teams to make rapid patient-centric deployment decisions. All of this is done by our experts before the kickoff meeting taking the burden off your teams. “Define” is the framework for cross-functional technology use in everything from simple registries to complex pivotal trials using sophisticated eCOA with telehealth visits.
Our Process
Our team is committed to understanding our client’s needs, anticipating, and mitigating challenges before they occur. That’s why our process starts from the moment of award to ensure, in most cases, that by the kickoff meeting we have completed the following activities:
- Analyzed the protocol for operational considerations for launching the study on THREAD platform
- Initiated the collection of sponsor feedback required for platform configuration
- Aligned on the participants, researchers, and data journey with a Journey Map
- Ensured all necessary documents are in place or in route for the critical path
- Initiated licensing of any eCOA to avoid timeline delays from third parties
- Produced an initial prototype of the research platform experience for client review
Patient Journey Mapping
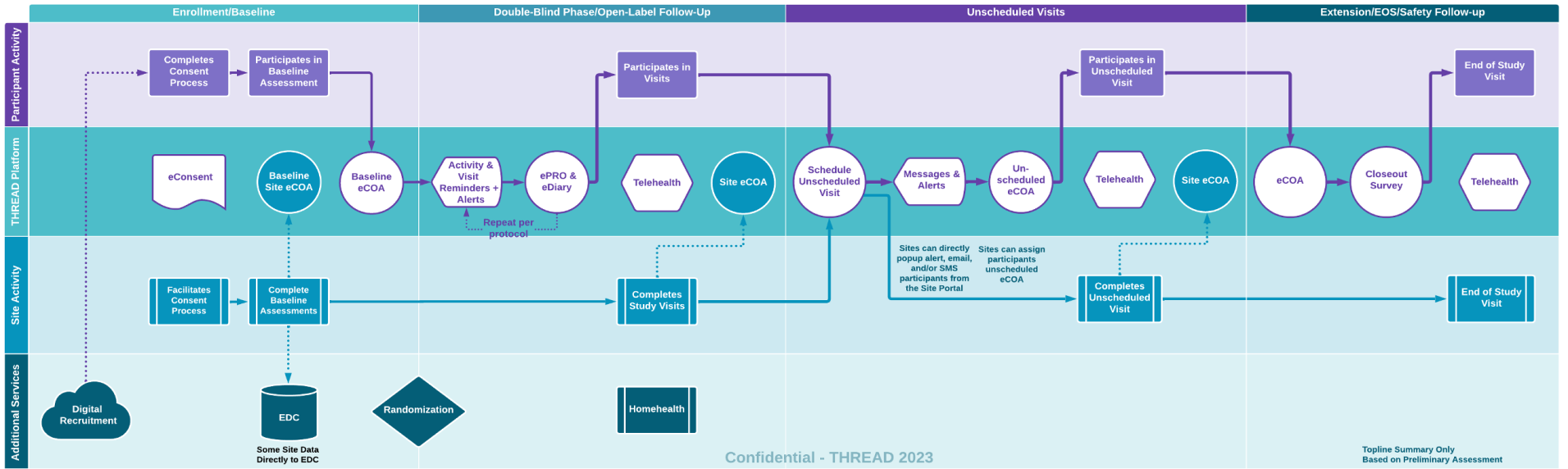
In this example the journey map summarizes the interplay between key stakeholders in your study and the data collected on THREAD. Prior to kick off our experts will create a more detailed journey map specific to your study to ensure researchers can manage and monitor the study, while participants engage, learn, and complete eCOA across the study lifecycle.
- Enrollment/Baseline Phase — Whether recruited onsite or remotely participant can onboard and eConsent in THREAD including screening and baseline eCOA from both participants and research sites.
- Double Blind Phase — During the primary research phases participants will receive reminders and alerts that align with the study protocol (SMS/popup/email). They will be able to complete their eDiaries. On visits with telehealth calls patients will receive a popup and complete the call with the site in Patient App. The sites will be able to simultaneously complete the telehealth call within the Site Portal while entering eCOA and eCRF data.
